UPGRADE OF HMI WITH ZENON SCADA: A COMPREHENSIVE SOLUTION FOR HASAN IN ITS GLOBAL EXPANSION PHASE
Hasan-Dermapharm is a joint venture between Hasan Pharmaceutical and the leading German pharmaceutical group, Dermapharm AG. Currently, the company is one of the leading entities in Vietnam’s pharmaceutical industry, having achieved WHO GMP, GLP, and GSP certifications granted by the Vietnam Drug Administration.
With the goal of expanding into international markets, Hasan-Dermapharm has embarked on building a new production facility aimed at meeting EU-GMP standards and complying with FDA CFR 21 Part 11 regulations. To achieve this, Hasan-Dermapharm entrusted Đức Phong with upgrading its HMI system to SCADA running on a panel PC for the PD-SPM-001 blister packing machine and the PD-TFM-001 effervescent tube filling machine.

Current Configuration and Issues
The primary issue with the current configuration of the blister packing machine (PD-SPM-001) and the effervescent tube filling machine (PD-TFM-001) is that the HMI interface only allows operators to control and operate the machines. As a result, there is no capability to ensure data integrity or traceability, which is essential for quality control of the pharmaceutical products produced by these machines. To comply with FDA and EU-GMP requirements, these machines need the capability to record and store data for generating traceability reports of the produced medications. Additionally, there must be features for user access control and management to ensure information security and data integrity.
DPTA’s Solution
DPTA’s proposed solution is to replace the current HMI screens on both machines with a panel PC running SCADA runtime programmed using COPA-DATA’s Zenon platform. Đức Phong selected Zenon as the foundation for developing the SCADA system due to its robust compatibility with a wide variety of terminal devices. COPA-DATA provides more than 300 drivers supporting most communication standards available in the market. Furthermore, Zenon has a proven track record of meeting FDA 21 CFR Part 11 requirements since the year 2000.
.png)
As per the customer’s requirements, in addition to retaining the original control features, DPTA will design and integrate four types of reports: Alarm Report, Batch Report, Batch Audit Trail Report, and Audit Trail Report, to facilitate traceability. Data integrity will be ensured through a variety of data storage options and a robust user management and hierarchy system. Additionally, DPTA has incorporated a batch lock feature that locks production batches when duplicate information is detected, tailored specifically to Hasan’s needs.
.png)
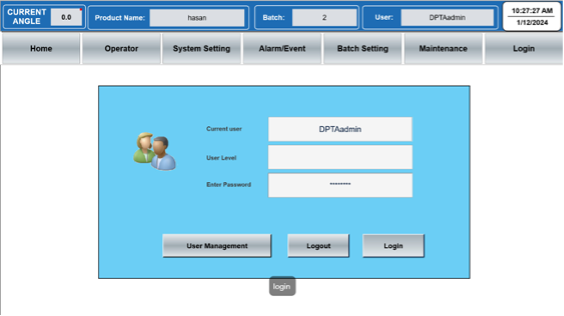
Achieved Results
With the upgrade solution provided by Đức Phong Technology and Automation JSC, Hasan’s blister packing machine and effervescent tube filling machine are now ready for production, fully compliant with international standards such as EU-GMP and FDA 21 CFR Part 11. This marks a significant milestone in Hasan-Dermapharm’s efforts to elevate its position in both domestic and international markets. A key contributor to this success is the robust compatibility and diverse international features offered by the Zenon SCADA software.
.png)
Bài viết liên quan
- UPGRADE OF KILIAN E150 TABLET PRESS: NEW CONTROL CABINET UTILIZING B&R’S HYPERVISOR TECHNOLOGY AND ZENON SCADA ENHANCES PRODUCTION EFFICIENCY
- UPGRADE OF KILIAN E150 TABLET PRESS: NEW CONTROL CABINET UTILIZING B&R’S HYPERVISOR TECHNOLOGY AND ZENON SCADA ENHANCES PRODUCTION EFFICIENCY
- SCADA SYSTEM AT INSEE THI VAI: A COMPREHENSIVE SOLUTION FROM DPTA
- ENHANCING EFFICIENCY AND PROVIDING COMPREHENSIVE SAFETY SOLUTIONS FOR CARGILL VIETNAM
- CARGILL TIEN GIANG & LONG AN BOOST OPERATIONAL EFFICIENCY WITH A COMPREHENSIVE PCS SYSTEM
- UPGRADE OF THE CONTROL SYSTEM FOR THE FETTE P2100 ROTARY TABLET PRESS
- SUCCESSFUL UPGRADE OF THE EXTRUDER AND CONDITIONER SYSTEM AT CARGILL DONG THAP


 Online:
Online:  During the day:
During the day:  In month:
In month:  Total access:
Total access: